Highlight
PD Assisted Catalytic Hydrogenation of Poly(styrene)
Achievement/Results
Palladium-based catalysts were investigated for hydrogenation of poly(styrene) (PS) to poly(vinylcyclohexane), a model polymer modification reaction. Catalyst support type, pore size, influence of a second metal, and preparation method on the activity and selectivity were all explored relative to a high dispersion Pd monometallic baseline catalyst. Results show such reactions in dilute solution (<10 wt% PS) are not internally diffusion-limited for any mesoporous (average pore sizes >2 nm) catalysts. The solvent choice had the greatest impact on activity; poorer solvents led to higher activity due to polymer chain collapse, resulting in a smaller effective chain radius. The calculated radius of gyration for PS in the bulk solution is 14 nm; clearly the polymer’s intrapore configuration differs greatly from bulk solution.
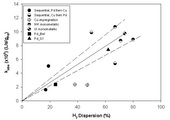
Intrinsic activity was quantified by turnover frequency on a per active metal site basis. Both mono- and bimetallic polymer hydrogenation catalysts fall in a narrow band of activities almost entirely determined by Pd dispersion (see graph below). Our results confirm that typical supported metal catalysts (on mesoporous supports) “work” for polymer modification reactions.
Address Goals
This is basic work to convert polystyrene, a commodity chemical with a well-known processing stream, to a fully hydrogenated form. The results add to our knowledge of the fundamental configurational and dynamical behavior of polymers when confined in porous substances. The student fellow graduated on time and was quickly snapped up by an industrial partner.