Highlight
Membrane Hemifusion Growth Kinetics
Achievement/Results
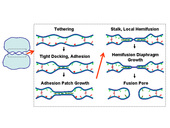
Membrane bilayers composed of amphiphilic phospholipids and proteins are soft materials of great importance to living cells. The fusion of membranes is an essential step in secretion and signaling processes and in intracellular material trafficking. SNAREs and other proteins are thought to mediate the following steps of the fusion pathway (see fig. 1): (i) membrane adhesion, (ii) hemifusion, and (iii) complete fusion.
Columbia University researchers Prof. Ben O’Shaughnessy and NSF IGERT Trainee Jason M. Warner have mathematically modeled the first two steps on the fusion pathway: adhesion and hemifusion. SNARE-mediated adhesion kinetics were described in the highlight from the previous IGERT reporting period; this highlight focuses on the model and results for hemifusion growth kinetics.
There is considerable evidence that hemifusion is an intermediate on the pathway to fusion. In the hemifused state the outer leaflets are fused while inner leaflets contact in the hemifusion diaphragm (HD) zone. Such a pathway, in which fusion is achieved in two steps, may advantageously involve lower kinetic barriers as compared to direct fusion when all four leaflets must be simultaneously reconnected.
The hemifused state is metastable: if a hemifusion diaphragm (HD) welding two vesicles grows beyond a critical size, membrane tension tends to expand it. In vivo , membrane tension may be assisted by force producing proteins. HD expansion may promote fusion. It has been proposed that fusion pores may typically form either within the HD, or preferentially along its highly stressed rim. Thus, increasing HD area and perimeter length may expedite fusion. In a different view, the possible off-pathway intermediate termed unrestricted hemifusion may involve extended HDs whose growth may disfavor pore formation by delocalizing the efforts of protein machines. In either case, HD growth is a central issue.
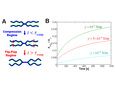
We modeled HD dynamics as follows. Growth is driven by the membrane tension – and opposed by the interleaflet tension – due to compression (expansion) of the outer (inner) membrane leaflets, which results as the HD expands. Two regimes of hemifusion growth were found (see fig. 2A): (i) the initial compression regime for small times, and (ii) the flip-flop regime for long times. Each regime has distinct kinetics.
In the compression regime (fig. 2A) the outer monolayer is progressively compressed relative to the inner monolayer to make room for the growing HD. The compression comes to a halt, and the HD stops growing when the lipid density in the outer monolayer has been elevated everywhere in the vesicle surface; the time for uniform compression to be achieved was found to be τcomp=(λ/k) Av determined by the vesicle membrane area Av, the interleaflet-friction drag coefficient λ and the monolayer compression modulus k. Because the monolayer stiffness is very large, the compression regime is short-lived: for typical vesicle sizes, τcomp << 1 s. Though the early compression regime is short, it is important because significant HD growth may occur. The final HD area was found to be Ahd = (1/2)(γ/k)Av which is large if tension is large. For example at high tensions close to lysis, the compression regime leads to HD area ~ 5% of the vesicle area. This prediction is consistent with the observations of Melikyan et al (J. Cell. Biol., 1995) who used osmotic pressure-induced membrane rupture to estimate that HD area was 3 − 5% of the total cell membrane area of red blood cells hemifused to hemagluttinin-expressing cells.
In the flip-flop regime (fig. 2A) interleaflet mass transfer by lipid flip-flop is required for further growth which proceeds at a much slower rate than in the compression regime. The long waiting time τ for lipid flipping (typically τ ~ 1 hr) and membrane tension determine the areal HD growth rate: dAhd/dt = (1/2)(γ/k) Av/τ.
Figure 2B shows predicted evolutions of relative HD area for several tension values. The early compression regime is so short on these timescales it corresponds in effect to the intercept at t = 0. Another important feature is included: as the HD grows (in the flip-flop regime) membrane tension relaxes and so growth rate slows. This is clearly exhibited by the curves in fig. 2B.
Address Goals
Combination of this theory with experimental results in the future will help understand the mechanisms of biological fusion. In particular, it will be illuminated how the fusion-protein machinery controls HD size on the pathway to fusion. Additionally, this work will shed light on the results from in vivo and in vitro experiments that were previously poorly understood and will motivate future experiments.
This highlight addresses the primary discovery outcome. This research addresses issues key to understanding biological fusion, and additionally, has direct application in biotechnologies (e.g. advanced drug delivery) and medicine (e.g. fighting HIV entry/infection via membrane fusion). Upon addition of these results to the research group’s public website following publication, the contents of this highlight will address the secondary learning outcome by expanding the scientific knowledge of all citizens.