Highlight
Designer Crystals for Sustainable Electronics
Achievement/Results
Working with a team of interdisciplinary scientists and engineers at Cornell University, IGERT Fellow Joshua Choi has developed a new technique to create “designer crystals” with the properties necessary for next-generation solar cells, transistors, and light emitting diodes.
Natural crystals are three-dimensional, periodic arrays of atoms. The arrangement of the atoms in these crystals greatly influences their optical and electrical properties. Different arrangements often have different properties. To mimic this natural control, nanotechnology researchers around the world are trying to pack nearly spherical nanometer-scale crystals or “quantum dots” into controlled, periodic arrays, thereby creating so-called artificial crystals with tunable properties. In much the same way that individual aluminum atoms have very different properties from an aluminum bar, these artificial crystals would have interesting new properties distinct from those of individual quantum dots. To accomplish this goal, scientists must understand and ultimately control the mechanisms that govern crystal formation at the nanoscale.
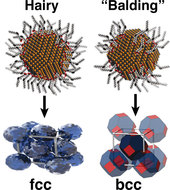
The Cornell research team synthesized six-nanometer-diameter lead sulfide quantum dots using inexpensive and scalable wet-chemistry methods. They then bonded different amounts of oleic acid — the basic ingredient of olive oil — to the surface of the nanocrystals, thereby creating “hairy dots.” Under the appropriate conditions, the hairy dots spontaneously formed crystals which were then studied by the powerful x-ray beams produced by the Cornell High Energy Synchrotron Source. Remarkably, the researchers found that the structure of the crystal could be changed by varying the amount of oleic acid (“hair”) on the dots. Very hairy dots formed face-centered cubic (fcc) crystals, whereas more sparsely covered dots formed body-centered cubic (bcc) crystals. When combined with theoretical calculations, these experiments provided new insights into the important role that surface chemistry plays in the growth of crystals at the nanoscale and suggested new techniques for controlling their growth and properties
The novel method of controlling the structure of nanocrystal arrays is simple, inexpensive, and suitable for industrial-scale mass production, taking researchers one step closer to their goal of designer materials for a sustainable future.
Choi, J. J., Bealing, C. R., Bian, K., Hughes, K. J., Zhang, W., Smilgies, D. M., Hennig, R. G., Engstrom, J. R., Hanrath, T. (2011). Controlling Nanocrystal Superlattice Symmetry and Shape-anisotropic Interactions Through Variable Ligand Surface Coverage. J Am Chem Soc, 133, 3131-3138.
Address Goals
Discovery: The development of new techniques for the self-assembly of nanocrystals enables the creation of “designer crystals” with the properties necessary for next-generation solar cells, transistors, and light emitting diodes. The novel method of controlling the structure of nanocrystal arrays is simple, inexpensive, and suitable for industrial-scale mass production, taking researchers one step closer to their goal of designer materials for a sustainable future.
Learning: This highly interdisciplinary and integrative research project required the sustained contributions of scientists and engineers with a wide range of talents and expertise. This project required contributions from surface chemists to enable the production of monolayer-thick films, from computational materials scientists to calculate the interactions of the nanocrystals, and from physicists to measure the structure of the assembled crystals. This interdisciplinary milieu provided an ideal training ground for next-generation scientists and engineers educated in cutting-edge nanoscience.