Highlight
Microtubule Motor Traffic Jams
Achievement/Results
In neurons, cellular material is primarily made in the cell body and must be transported to the ends of axons in order to maintain healthy axons. A disruption in this axonal transport process has been shown to result in the onset of neurodegenerative disease. Microtubule motor proteins are imperative for normal axonal transport. By simultaneously binding cargo and microtubule tracks within the axon, motor proteins can processively move cargo, such as proteins and organelles, from the cell body to the end of an axon. Kinesin-1 and cytoplasmic dynein are the key motor proteins involved in this process. Both use energy derived from ATP hydrolysis to power processive motion of cellular cargo. Kinesin-1 is responsible for anterograde transport, carrying cargo from the cell body to the end of the axon. Cytoplasmic dynein is responsible for retrograde transport, carrying cargo in the opposite direction, from the end of the axon back to the cell body for degradation. A complete understanding of how these motor proteins function is essential for understanding axonal transport, and thus the neurodegenerative diseases linked to problems in this process.
Numerous in vitro single molecule studies have been carried out to elucidate the mechanisms of these motor proteins. However, little work has been done to understand how motor proteins function in the cellular environment. Unlike a dilute, single molecule experiment, microtubules in cells are coated with other motor proteins and microtubule-associated proteins that could be potentially blocking the path for the processive motion of kinesin-1 and cytoplasmic dynein. How these motor proteins cope with such crowded environments remains unclear.
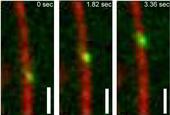
Leslie Conway, a Molecular and Cellular Biology ICE IGERT trainee working in the Ross lab (Physics), is part of a team investigating the properties of kinesin-1 in crowded conditions. Researchers work to mimic microtubule crowded conditions in vitro using increasing concentrations of unlabeled kinesin-1. Quantum dots, which have been shown previously to non-specifically bind kinesin-1, are added to kinesin-1 coated microtubules. Upon binding to kinesin-1 on microtubules, quantum dots act as cargo. Figure 1 illustrates a quantum dot labeled kinesin-1 walking along a microtubule. Adding quantum dots to microtubules with increasing concentrations of kinesin-1 allowed researchers to understand how cargo transport was affected by different levels of crowded conditions.
Single quantum dots were imaged using Total Internal Reflection Fluorescence (TIRF) Microscopy. These quantum dots were then tracked using a MATLAB program written by physics professor, Dr. Maria Kilfoil, and physics graduate student, Derek Wood. This program allowed Leslie to accurately determine and compare properties of the quantum dot cargoes, such as velocity, run length, association time, pause durations, and backwards motions in different degrees of crowded conditions.
From this work, Leslie found that as microtubules become more crowded with kinesin-1, the average run length and association time of transported cargoes increases. The average velocity of these cargoes decreases in more crowded conditions as they encounter more obstacles. She also observed that in more crowded conditions, while cargos pause more frequently, these pause durations actually become shorter in higher crowding. This could be due to the ability of cargos with multiple motors to switch protofilament tracks upon reaching an obstacle. In addition, she observed backwards motions of cargoes being transported by kinesin-1. These backwards motions occurred at a higher frequency in more crowded conditions. The team hypothesizes that these backwards motions occur when multiple motors are attached to a single cargo. When the cargo reaches an obstacle, the front kinesin-1 cannot proceed, and the cargo releases, resulting in a backwards rocking motion of the cargo, as a rear kinesin-1 attaches to the microtubule.
Address Goals
The primary goal of this work addresses discovery. This research has shed light on the fundamental topic of intracellular transport, a process essential for the understanding of numerous cellular events. A thorough understanding of how motor proteins function in a complex environment will help to develop a complete understanding of the properties of microtubule based motor transport in cells.