Highlight
High Pressure Particle Size Reduction (HPC and HPNC)
Achievement/Results
Production and Stabilization of Organic Crystalline Drug Nanoparticles using High Pressure Homogenization and Anti-solvent Crystallization
Graduate Students: Frank Romanski (Rutgers), Eric Jayjock (Rutgers), Xiangxin Meng (NJIT)
Project Coordinators: Dr. Fernano Muzzio (Rutgers), Dr. Somenath Mitra (NJIT), Dr. Silvina Tomassone
Recent research in the area of poorly water soluble drugs through creating nanoparticles has placed more emphasis on finding new methods of particle production. This collaboration focuses not only on the production of the particles, but on maintaining the particle size, shape, and characteristics to create a stabilized suspension of nanoparticles. Tried-and-true methods such as high pressure homogenization and liquid-antisolvent crystallization processes have been shown in the past to consistently produce submicron and nanoparticles of a narrow, unimodal, particle size distribution. Using processes that have been proven to work allows the research to focus on the immediate and long term stabilization of the particles within the liquid medium, rather than the creation of the particles.
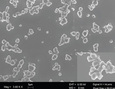
This study focuses on two methods of creating submicron and nano-particles. The first process is a “top down” approach utilizing communition called High Pressure Homogenization (HPH). In this process particles are forced through a very small piston gap (5-25 microns) at pressures of up to 30,000 psi. This process subjects the particles to extremely high shear and cavitation forces breaking the particles into smaller particles. The second method explores the “bottom-up” approach of forming the particles by liquid antisolvent crystallization with the aid of sonication to produce the small crystals. Despite knowledge that the respective techniques have proven successful in organic nanoparticle production, in order to succeed in this project both of these methods rely heavily on the collaborative effort to properly utilize surfactants to halt crystal growth and limit agglomeration between the high surface area nanoparticles. Many different surfactants and polymers have been tested in both stabilization experiments and in each process. Following production, the particles are then analyzed using several different analytical techniques including Scanning Electron Microscopy (SEM) and Dynamic Light Scattering (DLS). Samples are then re-examined over time for particle size and shape to monitor the possible growth and/or agglomeration.
The collaborations between the two universities have succeeded in narrowing down the massive pool of non-ionic surfactants, anionic surfactants, and block co-polymers to determine the most effective means of stabilizing a suspension. The two projects have since shifted towards using HPMC (hydroxyproplymethylcellulose) as a block co-polymer surfactant. This particular block co-polymer has shown extraordinary results in stabilizing suspensions for two examples of poorly water soluble drugs: Griseofulvin (anti-fungal) and Fenofibrate (anti-cholesterol). Traditional methods of particle synthesis rely largely on using high quantities of non-ionic and anionic surfactants. Unfortunately, using large quantities of non-ionic and anionic surfactants is not possible for the eventual goal of producing drug delivery systems that are safe for human consumption. However, using HPMC, considered to be an extremely safe polymer, the drug suspensions can be stabilized in a solution of water containing no other surfactants. Through joint effort the team has created two successful methods for creating organic crystalline nanoparticles that are stable enough to be stored and used for further processing into drug suspensions, strip-films, and gel capsules.
Address Goals
The primary goal of this research is for the discovery and advancement of technologies capable of delivering drugs to patients that are cheaper, easier to deliver, and more effective. Far too many drugs are immediately discredited by the industry due to poor water solubility. Through the use of two size reduction techniques this project explores methods of increasing the effective solubility and thus bioavailability of poorly water soluble drugs. The first method of creating the organic particles is through liquid antisolvent crystallization. The use of a variety of new surfactants and polymers in addition to sonication has shown a significant improvement to not only the production of the smaller particles but also to the stability of the suspension. The second method of creating organic crystalline nanoparticles is high pressure homogenization. This technique has proven to be an effective method of creating the stabilized suspension of particles in a method that can be scaled to an industrial size. Through the use of two different particle creation techniques the collaboration is quickly approaching the goal of practical drug delivery with poorly soluble drugs by using modifications to old technologies and the addition of new polymers and surfactants.
A second focus of the research stems from learning on a fundamental scientific level. This extremely diverse, collaborative project features subject matter from a wide array of sciences. Colloids and surface chemistry dominates the study of the particles on the nano-scale, while chemical and biomedical engineering principles are used to optimize and scale-up existing technologies. Finally, pharmaceutical engineering, pharmacy, and toxicology are all required to create not only a working drug delivery system, but one that is safe for human consumption on a world-wide scale. Through this collaboration of the sciences the project will soon produce drug delivery products that will improve the way of life for many people. By succeeding in creating a sound method for delivering poorly water soluble drugs we will effectively increase the pool of available drugs significantly and simultaneously reignite interest in previously discounted drugs.