Highlight
Novel Silica Nanoparticles Enhance Gene Delivery to the Spinal Cord: Potential to Control Pathological Pain
Achievement/Results
The NSF IGERT program on Integrating Nanotechnology with Cell Biology and Neuroscience (INCBN) at the University of New Mexico has provided the funding for the fellowship of Graduate Trainee Ellen Dengler, which has supported her involvement in research on the use of novel silica nanoparticles as a non-viral platform to deliver DNA and/or small molecules to the spinal cord as a means to control chronic neuropathic pain. This work is carried on in labs Ms. Dengler’s advisor Dr. Erin Milligan, Department of Neurosciences and co-advisor Dr. C. Jeff Brinker, Department of Chemical and Nuclear Engineering. This is an interdisciplinary effort involving neurobiologists and chemical and material engineers.
Chronic neuropathic pain (> 3 months) is characterized by pathological sensory processing that may include perception of light mechanical touch as painful, known as allodynia. Phagocytic immune cells (macrophage and dendritic cells) and glial cells (astrocytes and microglia) in the spinal cord and in the dorsal root ganglia (DRG) have emerged as key contributors to pathological pain. Upon activation, innate immune cells and glia release chemical messages, such as the pro-inflammatory cytokines, interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF) that mediate pathological pain states. The anti-inflammatory cytokine, interleukin-10 (IL-10), is endogenously produced and inhibits the production and activity of IL-1, IL-6, and TNFalpha. Pain relief produced in a well-characterized rat model of neuropathic pain is observed for approximately 3 months following a peri-spinal injection (intrathecal; i.t.) of naked plasmid DNA encoding the IL-10 gene (pDNA-IL-10). However, two i.t. injections at very high doses (100 µg and 25 µg) are required to produce enduring pain control.
The goal of this project is to improve dosage formulations of pDNA-IL-10 for future clinical applications to control neuropathic pain. By encapsulating pDNA-IL-10 in novel silica nanoparticles, “protocells”, gene transfer to the spinal cord may be optimized, thereby reducing dosage requirements. Protocells are composed of a silica core, which can support a fatty acid containing lipid bi-layer covering its surface. The core has an evenly sized pore (mesoporous) structure, making it ideal for loading small molecules and genes of interest. In addition, surface charge interactions allow loading of other, larger molecules, such as DNA.
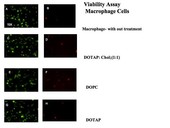
The first research focus was to determine if the particles would be tolerated in mammalian cell culture, as assessed by a live/dead viability cell assay (Invitrogen, Inc.). A mouse macrophage cell line (RAW264.7) was chosen because it closely resembles the cell type suspected of taking up pDNA-IL-10. Results revealed that over 95% of cells remained alive after 72 hours following treatment with protocells (Figure 1).
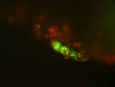
Given the particles failed to induce overt toxicity in cell culture, their potential toxicity and biodistribution was examined in vivo. Protocells supporting either a positively or neutrally charged lipid bi-layer were loaded with green fluorescent-tagged DNA sequences. They were then injected into the rat i.t. space of the spinal cord surrounding neurons that carry pain messages. Resident macrophage cells in the meninges were found to take up protocells. Meninges are made of several thin layers of tissues that surround the spinal cord. Pain neurons inside the spinal cord were not observed to take up the protocells (Figure 2).
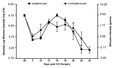
To determine that the particles were not toxic in vivo, rat behavioral responses to light mechanical touch were evaluated before (baseline; BL) and after i.t. injection. Briefly, withdrawal responses to a light touch applied to the left and right hind paws by a series of calibrated monofilaments (<0.5-15 g) were assessed (von Frey test). At BL, under healthy conditions prior to i.t. injections, rats respond normally at ~10-15 g of touch stimuli. However, under conditions that lead to spinal glial activation and proinflammatory signaling, rats begin to respond to <0.5 g. Thus, these responses to very light tactile stimuli reflect proinflammatory events in the spinal cord. In stark contrast, following i.t. injection of protocells, animals experienced no adverse behavioral changes. Sensory responses did not vary from baseline; the animals gained weight normally, and were observed as having no change in their normal grooming and exploratory behavior (Figure 3).
Interestingly, following the i.t. injections, anatomical distribution of the protocells in the spinal canal varied depending on surface charge of the lipid supported by the silica core. Those supporting DOTAP: Cholesterol, which have a positively charged surface, remained locally at the injection site. Those supporting DOPC, which have a neutrally charged surface, spread over a broader range, including not only the lumbar & cervical spinal cord, but also the brain. These differences in biodistribution offer exciting potential for drug delivery. For example, under some pathological conditions, it might be ideal to deliver drug or gene cargo locally, as in the neuropathic pain produced by sciatic nerve damage. For different conditions where a larger portion of the spinal cord is affected, such as in multiple sclerosis, it would be advantageous to treat the entire spinal canal. By varying the lipid and/or the surface chemistry of the protocell, one can specifically target the localization (biodistribution) of spinal drug delivery. Results demonstrate a bilateral (ipsilateral and contralateral) reduction of pain for almost three weeks (Figure 4).
Address Goals
We chose to explore the use of protocells as a non-viral pDNA-IL-10 delivery platform to treat neuropathic pain. This gene drives expression of the IL-10 protein, which is known to reduce neuropathic pain following unilateral sciatic nerve damage in rats. In a pilot study, neuropathic rats were given i.t. pDNA IL-10 loaded onto protocells. Results demonstrated a bilateral (ipsilateral and contralateral) reduction of pain for almost three weeks (Figure 4).
We hope to extend this line of research and continue other studies to explore the amazing potential of protocells to be used in targeted drug and gene delivery. By varying its chemistry, the silica core can be tuned to release cargo in synchrony with different cellular events in the meninges, and the lipid coating can be bioconjugated to small ligands for receptor-mediated cell targeting in the spinal cord. These qualities offer a unique potential to simultaneously deliver both drug and gene therapies to treat not only pain but many other pathologies such as cancer and neurological disease.